Pakistan has succeeded in developing and approving Pakistan’s first PakVac Vaccine for COVID-19. This amazing feat was achieved with the help of Cansino Bio from China. This news was shared with the rest of the world after the vaccine was tested rigorously for quality control and effectiveness in fighting the coronavirus.
The vaccine went through many tests and trials before it has been passed to be used to fight against COVID-19 in Pakistan. With the availability of PakVac, it will help the Pakistani health sector to minimize their dependance on vaccines from other countries.
PakVac Now Approved
This is a great breakthrough for the healthcare community and the professionals. Faisal Sultan, Special Assistant to the Prime Minister for National Health Services, Regulation & Coordination shared his congratulations and good news with the public via his twitter.
In his tweet, he said, “Congratulations to the NIH (National Institute of Health) Pak team and its leadership for successful fill/finish (from concentrate) of the Cansino vaccine with the help of Cansino Bio Inc. China.” He also mentioned that the availability of the Pakistani vaccine will help with increasing vaccine supply for Pakistanis.
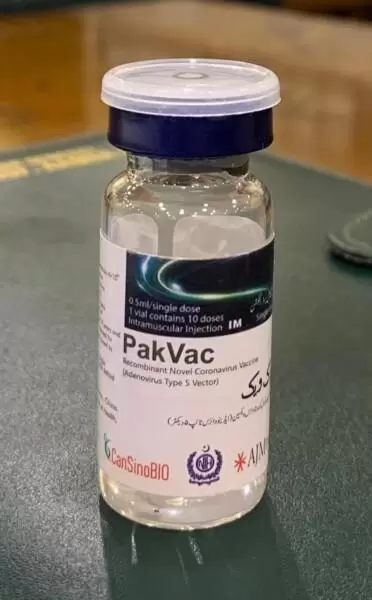
An official from the Ministry of National Health Services shared that the National Health Services will be able to produce 3 million doses per month, which is a good number to start with. In the meantime, the National Health Services announced that China’s single dose vaccine called CanSino will be available for all citizens by the end of May.
The statement said, “The first batch of bulk CanSino vaccine is being processed at the National Institute of Health’s plant, which was set up for this purpose last month, and a specially trained team is working on it.”
The raw material for the PakVac will reach Pakistan by early May and the production of the vaccination will begin immediately. If all goes well, we may have the vaccine ready to put into the arms of citizens by June.
